 Back Back |
Next
|
Nature
of Light and Matter
-
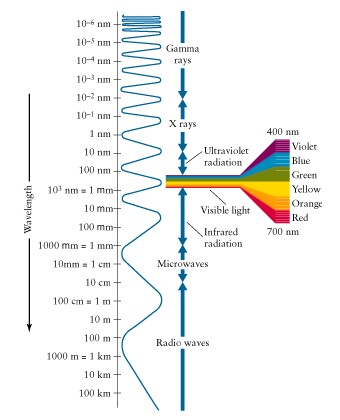
Electromagnetic
radiation
-
Long
wavelength (red) low energy
-
short
wavelength (blue) high energy
-
Frequency
(n) to wavelength
(l)
n =
c/l
-
Frequency
(n) to energy
(E):
E
=
hn
-
c
= speed of light = 3 x 108 m/s absolute speed limit
-
h
= Planck's constant = 4.135 x 1015 eV s
-
h
= 6.626 x 10-34 J s (value given on final)
-
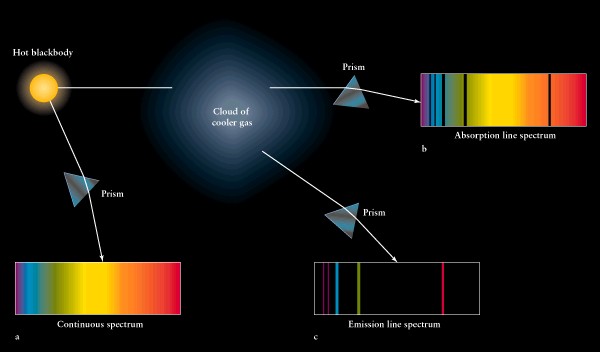
Each
element or molecule has its own unique set of spectral lines
-
recall
spectrum tubes (neon lights)
-
USING
SPECTRAL LINES, WE IDENTIFY ELEMENTS AND MOLECULES ON OTHER PLANETS, STARS,
ETC.
-
study
of spectra is "spectroscopy"
-
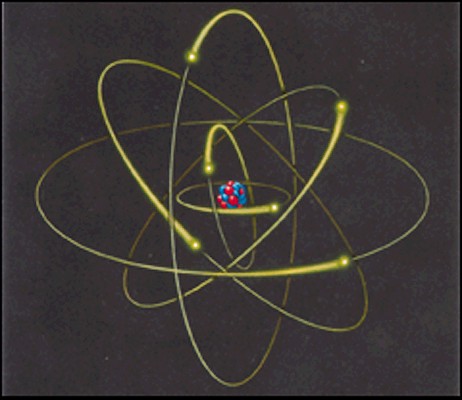
An
atom contains a dense nucleus and is surrounded by electrons
 Back Back |
Next
|


