 Back Back |
Next
|
The
nature of matter
-
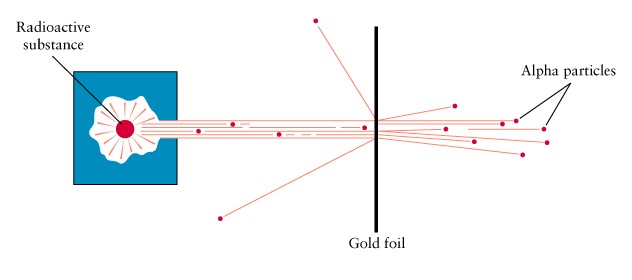
In 1910 Ernst Rutherford
beamed alpha particles at thin gold foil
-
thin gold foil like tissue
paper
-
alpha particles like bullets
-
Some alpha particles bounced
back!
-
Rutherford concluded that
there must be very dense things in the foil somewhere
-
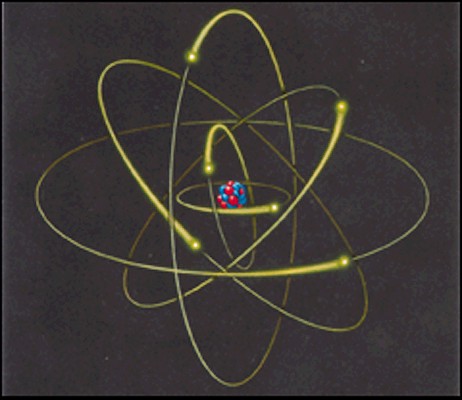
Neils Bohr worked out a model
of the Hydrogen atom shortly thereafter
-
one proton at the center
-
one electron orbiting in
certain
allowed orbits
-
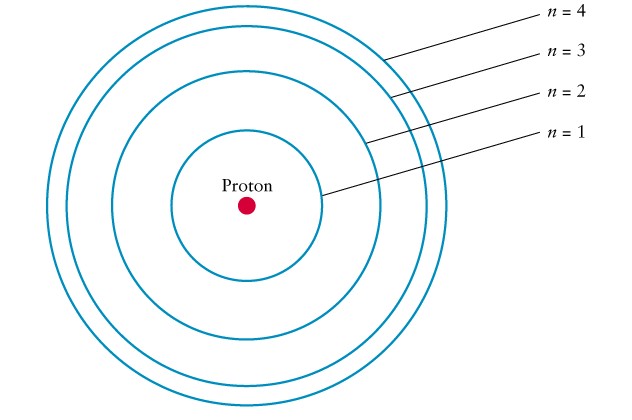
Quantum mechanics
predicts the positions of these orbits
-
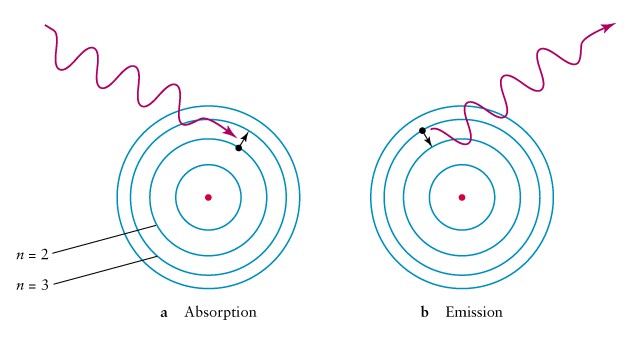
Emission line: electron jumps
to lower orbit
-
Falling in gives up energy
-
Absorption line: electron
jumps to a higher orbit
-
climbing out takes energy
-
Analogous processes in other
atoms and molecules
Rutherford's
experiement
Model
of atom
Model
of electron orbits

Photon
Absorption and Emission
Term
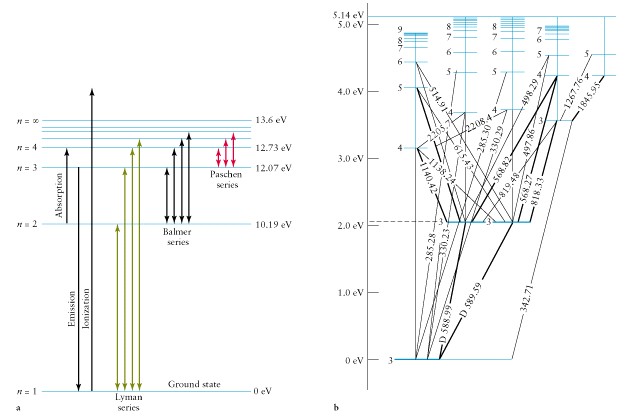
diagrams for Hydrogen and Sodium
 Back Back |
Next
|





