
 Our group is involved in the study of x-rays originating from the interstellar medium. Specifically, we study the diffuse x-ray background and its origins. To study x-rays we use quantum calorimeters. Quantum calorimeters are small devices that measure the thermal energy deposited when an x-ray strikes a detector. With these measurements we are able to characterize x-rays and thereby gain information about their origin.
Our group is involved in the study of x-rays originating from the interstellar medium. Specifically, we study the diffuse x-ray background and its origins. To study x-rays we use quantum calorimeters. Quantum calorimeters are small devices that measure the thermal energy deposited when an x-ray strikes a detector. With these measurements we are able to characterize x-rays and thereby gain information about their origin.
Our group's goal is therefore two-fold, first,studying of the x-ray background and its origins; second, developing better microcalorimeters to aid us in our study of x-rays.
Introduction to X-Rays
X-Rays and the E&M Spectrum
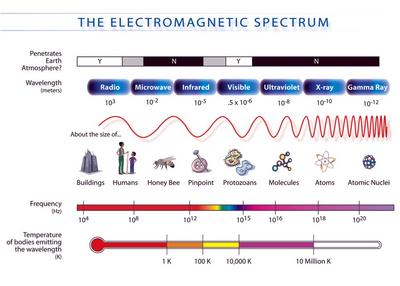 X-rays were first recognized as a new type of radiation by Wilhelm Conrad Röntgen, a German physicist in 1895. An x-ray is a type of electromagnetic radiation with an evergy range of .1 - 100 keV, a wavelength range of 10 - 0.01 nm and a frequency range of 30 - 30,000 PHz.
X-rays were first recognized as a new type of radiation by Wilhelm Conrad Röntgen, a German physicist in 1895. An x-ray is a type of electromagnetic radiation with an evergy range of .1 - 100 keV, a wavelength range of 10 - 0.01 nm and a frequency range of 30 - 30,000 PHz.
What Makes X-Rays?
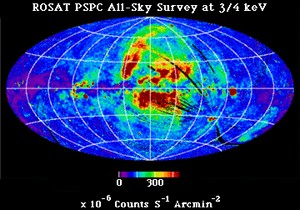 X-rays can be produced by a number of different processes: they can be emitted by hot gas, be a product of solar wind interactions , and emitted when an electron falls an energy level anlong with a number of other ways. The UW X-Ray Astrophysics lab focuses mostly on the three named processes and is working on figuring out their origins.
X-rays can be produced by a number of different processes: they can be emitted by hot gas, be a product of solar wind interactions , and emitted when an electron falls an energy level anlong with a number of other ways. The UW X-Ray Astrophysics lab focuses mostly on the three named processes and is working on figuring out their origins.
Difficulty in Studying X-Rays
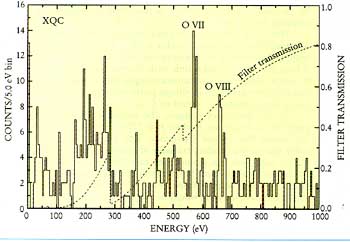 Unlike visible light, we can't observe X-rays by just looking at the sky. The detectors used to research X-rays gather data on the amount of energy of each X-ray photon, which can be measured with great precision with a microcalorimeter. This data is then interpreted into a spectrum and graphed. The spectra can be analyzed to find numerous new data about the X-rays that had been observed. Unfortunately, the earth's atmosphere abosorbs these high-energy rays, hindering astronomers' attempts to learn their secrets. To see X-rays at all, it is necessary to be above 90% of the earth's atmoshere; and to detect X-rays in the band where sources are most prominent, all but one millionth of the atmosphere must be below the instrument. In order to run worthwhile experiments, rockets are required to lift X-ray detectors above earth's atmosphere. It is not a trivial matter to build instruments that are large enough to be sensitive, yet small enough to fit within a rocket. Instruments have to withstand the rigors of launch but also operate in a vacuum.
Unlike visible light, we can't observe X-rays by just looking at the sky. The detectors used to research X-rays gather data on the amount of energy of each X-ray photon, which can be measured with great precision with a microcalorimeter. This data is then interpreted into a spectrum and graphed. The spectra can be analyzed to find numerous new data about the X-rays that had been observed. Unfortunately, the earth's atmosphere abosorbs these high-energy rays, hindering astronomers' attempts to learn their secrets. To see X-rays at all, it is necessary to be above 90% of the earth's atmoshere; and to detect X-rays in the band where sources are most prominent, all but one millionth of the atmosphere must be below the instrument. In order to run worthwhile experiments, rockets are required to lift X-ray detectors above earth's atmosphere. It is not a trivial matter to build instruments that are large enough to be sensitive, yet small enough to fit within a rocket. Instruments have to withstand the rigors of launch but also operate in a vacuum.
Microcalorimetry
What is Microcalorimetry?
 Your opponent's serve was almost perfect, but you vigorously returned it beyond his outstretched raquet to win the point. Now the tennis ball sits wedged in the chain-link fence around the court. What happened to the ball's kinetic energy? It has gone to heat the fence, and you realize that if the fence were quite a bit colder, you might be able to measure that heat and determine just how energetic your swing really was. Calorimetry has been a standard measurement technique since James Joule and Julius von Mayer independently concluded, about 150 years ago, that heat is a form of energy. But only in the past 15 years or so has calorimetry been applied, at millikelvin temperatures, to the measurement of the energy of the individual photons and particles with exquisite sensitivity. A microcalorimeter is used to measure the energy of a single photon. X-ray astrophysics is one of the fields where most of the efforts in the development of cryogenic microcalorimeters are spent. The first microcalorimeters for X-ray astronomy were developed by the University of Wisconsin / NASA Goddard Space Flight Center collaboration.
Your opponent's serve was almost perfect, but you vigorously returned it beyond his outstretched raquet to win the point. Now the tennis ball sits wedged in the chain-link fence around the court. What happened to the ball's kinetic energy? It has gone to heat the fence, and you realize that if the fence were quite a bit colder, you might be able to measure that heat and determine just how energetic your swing really was. Calorimetry has been a standard measurement technique since James Joule and Julius von Mayer independently concluded, about 150 years ago, that heat is a form of energy. But only in the past 15 years or so has calorimetry been applied, at millikelvin temperatures, to the measurement of the energy of the individual photons and particles with exquisite sensitivity. A microcalorimeter is used to measure the energy of a single photon. X-ray astrophysics is one of the fields where most of the efforts in the development of cryogenic microcalorimeters are spent. The first microcalorimeters for X-ray astronomy were developed by the University of Wisconsin / NASA Goddard Space Flight Center collaboration.
Microcalorimeters were first employed on a sounding-rocket experiment (the X-ray quantum calorimeter - XQC) that had an array of microcalorimeters for the study of X-rays from the interstellar medium. Much effort now goes into creating second generation detectors capable of improving this performance.
What They Look Like
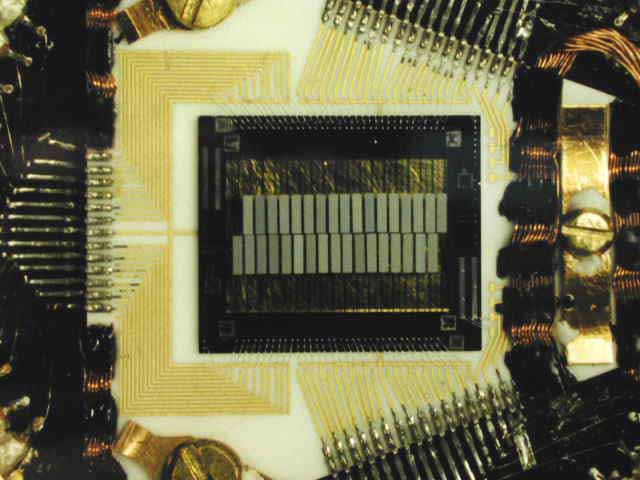
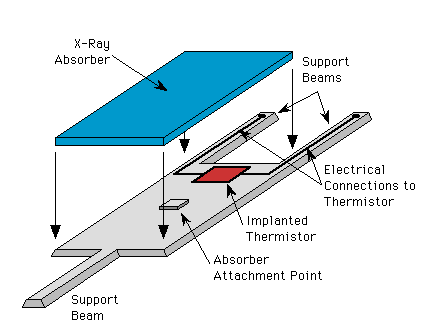 Microcalorimeters are made up of three basic components: an absorber, a thermometer and a weak thermal link. The thermometer is extremely sensitive that works by sitting at the sharp transition temperature of a superconductor. To know more about the future of these thermometers read about Transition Edge Sensor (TES).
Microcalorimeters are made up of three basic components: an absorber, a thermometer and a weak thermal link. The thermometer is extremely sensitive that works by sitting at the sharp transition temperature of a superconductor. To know more about the future of these thermometers read about Transition Edge Sensor (TES).
An X-ray calorimeter basically consists of a thermal mass to absorb incident X-ray photons, a thermometer to measure the resulting temperature rise, and a weak link to a low-temperature heat sink that provides the thermal isolation needed to sense a temperature change.
Why Do Microcalorimeters Need to Be So Cold?
 The calorimeter should operate at a low temperature so that the energy deposited is large relative to the overall temperature of the . In order to effectively measure the temperature change caused by one photon, the thermometer must be sensitive and thus cold. The absorber needs to have a low heat capacity so that the change in energy can be measured and must also reproducibly and efficiently distribute the energy of the initial photon across a thermal distribution of photons.
The calorimeter should operate at a low temperature so that the energy deposited is large relative to the overall temperature of the . In order to effectively measure the temperature change caused by one photon, the thermometer must be sensitive and thus cold. The absorber needs to have a low heat capacity so that the change in energy can be measured and must also reproducibly and efficiently distribute the energy of the initial photon across a thermal distribution of photons.
Requirements for a good microcalorimeter are an absorber with a small heat capacity able to convert the radiation from one photon into thermal energy quickly and with high efficiency, and a sensor with low heat capacity and high sensitivity to temperature variations.

